How to balance pool chemicals without using test strips is a question many pool owners ask. While test strips offer a quick assessment, learning to balance your pool’s chemistry using other methods provides a deeper understanding of your pool’s needs and can lead to better long-term maintenance. This guide explores visual cues, alternative testing methods, and predictive strategies to help you achieve a perfectly balanced pool, all without relying on those handy, but sometimes inaccurate, test strips.
We’ll delve into the crucial roles of chlorine, alkalinity, and pH, explaining how imbalances manifest visually. You’ll discover how to use liquid reagent test kits for more precise measurements, learn to create a proactive maintenance schedule, and even explore advanced techniques like calculating chemical additions based on your pool’s volume. By the end, you’ll feel confident in maintaining your pool’s chemical balance, even without the convenience of test strips.
Understanding Pool Chemistry Basics
Keeping your pool water sparkling and safe for swimming requires a basic understanding of pool chemistry. Maintaining the correct balance of chlorine, alkalinity, and pH is crucial for a healthy and enjoyable swimming experience. Improper chemical balance can lead to cloudy water, irritated skin and eyes, damage to pool surfaces, and even the growth of harmful algae.Maintaining the proper balance of pool chemicals involves understanding the role each plays in the overall health of your pool.
The three key players are chlorine, alkalinity, and pH. These elements interact with each other, and an imbalance in one can significantly affect the others.
Chlorine’s Role in Pool Sanitation
Chlorine is the primary sanitizer in most swimming pools. It works by killing bacteria, viruses, and other harmful microorganisms that can contaminate the water. Without sufficient chlorine, your pool becomes a breeding ground for these pathogens, posing a health risk to swimmers. Chlorine levels should be maintained within a specific range to be effective but not overly harsh on swimmers or pool equipment.
Too little chlorine leads to contamination, while excessive chlorine can irritate skin and eyes and damage pool surfaces.
Alkalinity’s Influence on pH Stability, How to balance pool chemicals without using test strips
Alkalinity acts as a buffer, helping to stabilize the pH of your pool water. It resists large swings in pH, preventing sudden and drastic changes that can harm both swimmers and the pool’s structure. Maintaining proper alkalinity prevents the need for frequent pH adjustments. Low alkalinity can cause the pH to fluctuate wildly, while high alkalinity can lead to cloudy water and scaling on pool surfaces.
pH Level and Its Impact on Pool Water
pH measures the acidity or basicity of your pool water. It’s expressed on a scale of 0 to 14, with 7 being neutral. Pool water should ideally be slightly alkaline, within a specific range. An imbalanced pH can affect the effectiveness of chlorine, irritate swimmers, and damage pool surfaces. Low pH (acidic) can corrode pool surfaces, while high pH (alkaline) can cause scaling and cloudiness.
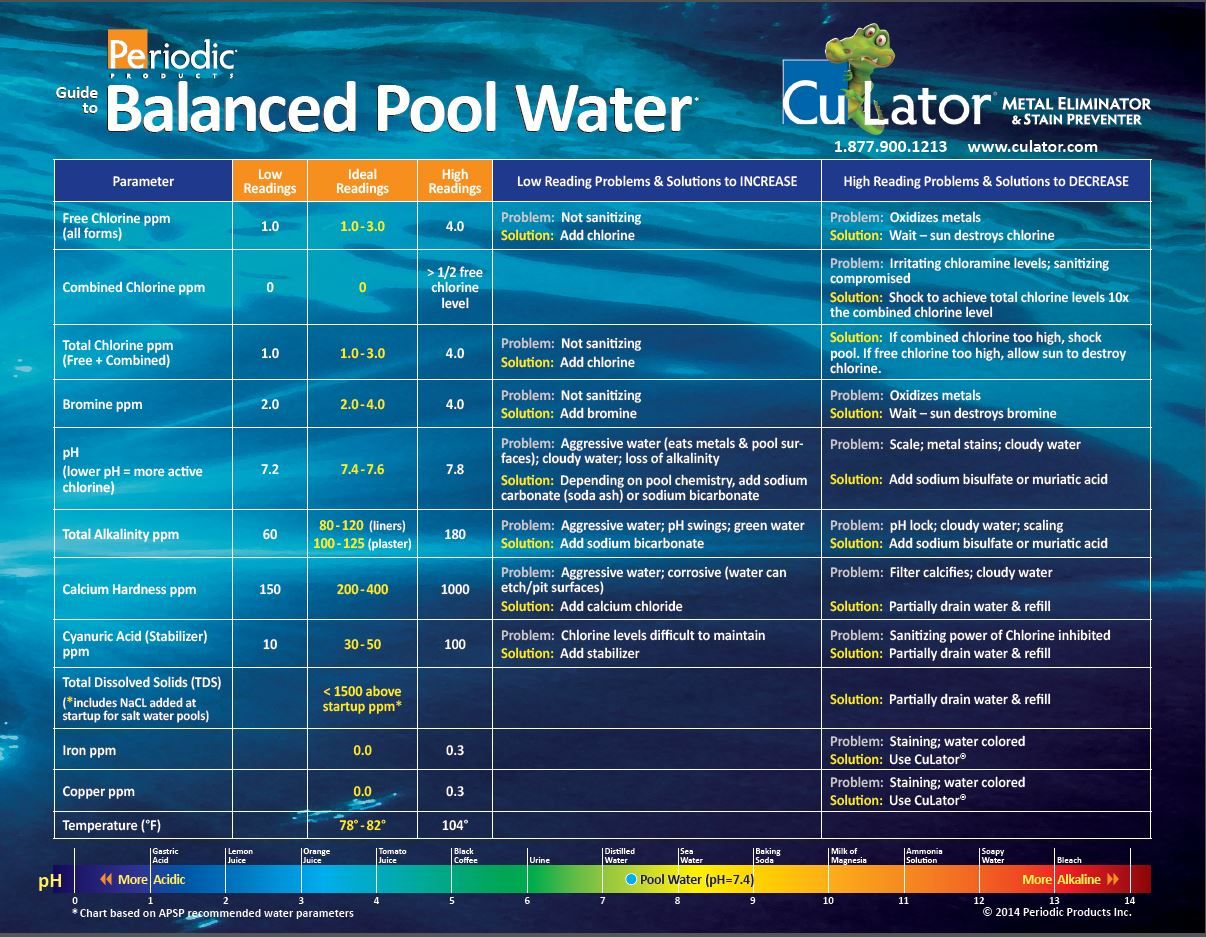
Ideal Chemical Ranges for Balanced Pool Water
Maintaining the correct balance of these chemicals is vital. Here’s a summary of the ideal ranges:
- Chlorine: 1.0 – 3.0 ppm (parts per million)
- Alkalinity: 80 – 120 ppm
- pH: 7.2 – 7.8
These ranges are generally accepted guidelines, and slight variations might be acceptable depending on factors like water temperature and bather load. Always consult your pool’s specific instructions or a professional for precise recommendations.
Effects of Imbalanced Pool Chemicals
The consequences of imbalanced pool chemicals are numerous and can significantly impact both the water quality and the pool’s structure. For instance, low chlorine levels can lead to algae blooms, resulting in green, cloudy water, while high chlorine levels can cause eye and skin irritation. Low pH can corrode plaster and metal surfaces, whereas high pH leads to scaling and cloudy water.
Alkalinity imbalances cause pH fluctuations, further exacerbating these problems. A consistently imbalanced pool will require more frequent and extensive chemical adjustments, increasing maintenance costs and potentially shortening the lifespan of pool components. In extreme cases, severe imbalances can necessitate a complete water drain and refill.
Visual Indicators of Imbalance
Source: pinimg.com
Knowing your pool’s chemistry without test strips relies heavily on observation. Visual cues, while not precise, can alert you to potential imbalances and guide you towards corrective actions. These observations should be coupled with your understanding of basic pool chemistry to accurately diagnose and address the issue.
Recognizing these visual indicators can help you avoid significant problems and keep your pool clean, clear, and safe for swimming.
Chlorine Imbalance Indicators
High chlorine levels often produce a strong, pungent chlorine smell, far beyond the faint, fresh scent you expect. The water might also appear slightly hazy or cloudy, even if other factors are balanced. Low chlorine levels, on the other hand, are indicated by the absence of that characteristic chlorine smell and potentially by the presence of algae, which appears as green, brown, or black discoloration and/or slime in the water.
Cloudy water could also suggest a lack of chlorine, alongside other possible chemical imbalances.
pH Imbalance Indicators
High pH levels can lead to scaling, which appears as white, chalky deposits on pool surfaces, particularly around the waterline and on pool equipment. The water may also feel slippery. Low pH, conversely, results in corrosion of metal components, like ladders, light fixtures, and pumps. You might notice rust stains or pitting on these surfaces. Additionally, low pH can cause eye irritation for swimmers.
Alkalinity Imbalance Indicators
Issues with total alkalinity often manifest as cloudy water, even if chlorine and pH are within acceptable ranges. Another key indicator is frequent fluctuations in pH. Alkalinity acts as a buffer, stabilizing pH. If alkalinity is low, the pH will swing wildly, becoming difficult to maintain. High alkalinity also contributes to scaling.
Visual Indicators Summary Table
| Chemical Imbalance | Visual Cue | Impact on Pool | Recommended Action |
|---|---|---|---|
| High Chlorine | Strong chlorine odor, hazy water | Eye irritation, potential damage to pool surfaces | Dilute with water, add a chlorine neutralizer (thiosulfate). |
| Low Chlorine | Lack of chlorine odor, algae growth, cloudy water | Unhygienic swimming conditions, algae blooms | Add chlorine, according to pool size and instructions. |
| High pH | Scaling (white deposits), slippery water | Scaling on pool surfaces, reduced sanitizer effectiveness | Add a pH decreaser (acid). |
| Low pH | Corrosion of metal parts, irritated eyes | Damage to pool equipment, eye irritation | Add a pH increaser (alkali). |
| Low Total Alkalinity | Cloudy water, frequent pH fluctuations | Unstable pH, difficulty maintaining balance | Add alkalinity increaser. |
| High Total Alkalinity | Scaling (white deposits), cloudy water | Scaling on pool surfaces, difficulty maintaining pH | Dilute with water, partially drain and refill the pool (if significant). |
Alternative Balancing Methods
Test strips offer a convenient way to check your pool’s chemical balance, but liquid reagent test kits provide a potentially more accurate and detailed analysis. These kits use chemical reactions to determine the levels of various chemicals in your pool water, offering a more precise understanding of your pool’s chemistry.Using Liquid Reagent Test Kits: A Step-by-Step GuideLiquid reagent tests offer a more precise measurement of your pool’s chemical balance compared to test strips.
These kits typically involve adding specific amounts of liquid reagents to water samples from your pool, resulting in color changes that are then compared to a color chart provided with the kit. This color comparison determines the concentration of each chemical.
Liquid Reagent Test Kit Procedure and Safety
Before beginning, always ensure you’re working in a well-ventilated area, wearing appropriate safety goggles to protect your eyes from splashes. Gather all necessary materials: the test kit, clean sample containers, a measuring device (typically a syringe or dropper), and the provided color chart. Carefully follow the instructions provided with your specific kit. Generally, this involves adding a precise amount of pool water to a test tube, then adding the specified liquid reagent(s) in the correct order and amounts.
After adding each reagent, gently swirl or mix the solution to ensure thorough reaction. Observe the color change and compare it to the color chart to determine the concentration of the chemical being tested. Remember to always dispose of used reagents and samples responsibly, according to the kit’s instructions. Never mix different reagents together unless specifically instructed to do so by the kit’s instructions, as unexpected and potentially dangerous reactions can occur.
Accuracy Comparison: Liquid Reagents vs. Test Strips
Liquid reagent tests generally offer greater accuracy than test strips. Test strips are convenient but can be less precise due to the subjective nature of color interpretation. The color changes in liquid reagent tests are often more pronounced and easier to compare against the color chart, reducing the potential for misinterpretation. The results from a liquid reagent test kit will typically provide a numerical value (e.g., ppm for chlorine or alkalinity), providing a more precise reading than the qualitative ranges (e.g., low, medium, high) typically provided by test strips.
However, even with liquid reagent tests, user error can still affect the accuracy of the results, emphasizing the importance of following instructions carefully.
Advantages and Disadvantages of Different Pool Test Kits
Several types of pool test kits are available, each with its own advantages and disadvantages. Factors to consider include cost, ease of use, accuracy, and the range of chemicals tested.
| Test Kit Type | Advantages | Disadvantages |
|---|---|---|
| Liquid Reagent Kits | High accuracy, numerical results, comprehensive testing options | More expensive, requires more steps, potentially more time-consuming |
| Test Strips | Convenient, inexpensive, quick results | Lower accuracy, subjective color interpretation, limited testing range |
| Digital Test Kits | High accuracy, easy to read digital display, often includes multiple chemical tests | Most expensive, requires batteries, may require calibration |
Predictive Balancing Strategies
Predicting your pool’s chemical needs allows for proactive maintenance, minimizing the risk of imbalances and ensuring a consistently enjoyable swimming experience. This approach moves beyond reactive adjustments based on test results, utilizing knowledge of your pool, its usage, and environmental factors to anticipate chemical needs. By implementing a predictive strategy, you can maintain a healthy pool with less frequent, intensive interventions.Regular chemical additions, scheduled proactively, are more effective than sporadic treatments.
This strategy is especially valuable in preventing significant imbalances that require more drastic corrective measures. A well-designed schedule considers several key variables, enabling you to fine-tune your approach for optimal results.
Weekly Pool Maintenance Schedule
A sample weekly schedule provides a baseline; however, it’s crucial to adapt it based on your specific pool’s characteristics and usage. Factors such as pool size, bather load, rainfall, and sunlight exposure significantly influence chemical consumption and evaporation rates.
| Day | Task | Chemical Addition (Example – Adjust based on your pool’s needs) | Notes |
|---|---|---|---|
| Monday | Visual Inspection | None (Observe water clarity, debris) | Check for algae growth, cloudy water, or unusual debris. |
| Tuesday | Skimming & Brushing | None | Remove leaves, insects, and other debris. Brush the pool walls and floor. |
| Wednesday | Add Chlorine | 1-2 tablets (2000 gallon pool, adjust accordingly) | Maintain a consistent chlorine level (consult a pool professional for guidance). |
| Thursday | Check pH and Alkalinity | Adjust pH and alkalinity as needed. (This example requires test strips or a testing kit. Visual cues alone are insufficient for accurate adjustment). | Maintain ideal pH and alkalinity ranges (7.2-7.8 for pH, 80-120 ppm for alkalinity). |
| Friday | Backwash Filter | None | Clean the filter to maintain optimal water clarity. |
| Saturday | Add Algaecide (if needed) | As directed on product label. | Prevent algae growth, particularly during periods of high heat and sunlight. |
| Sunday | Visual Inspection & Water Level Check | Add water as needed to maintain proper water level. | Check for any issues and replenish water lost due to evaporation or splashing. |
Adjusting Chemical Additions Based on Visual Cues
While precise chemical balancing requires testing, visual cues offer valuable insights. Clear, sparkling water generally indicates a well-balanced pool. Conversely, cloudy water, algae growth, or discoloration suggests an imbalance.For instance, cloudy water might indicate insufficient chlorine or a pH imbalance. Algae growth is a clear sign of insufficient chlorine or an imbalance in other chemicals. Discoloration, such as green or brown hues, directly points to algae problems.
These visual indicators guide adjustments to your schedule. If you notice cloudy water after your Wednesday chlorine addition, you may need to increase the amount or frequency of chlorine addition. Similarly, if algae appear despite regular chlorine additions, you may need to increase the frequency of algaecide application or consider a more powerful algaecide. Remember that visual cues are aids; for precise adjustments, testing remains necessary.
Advanced Techniques for Experienced Pool Owners
Maintaining perfect pool chemistry without test strips requires a deeper understanding of pool dynamics and precise calculations. This section delves into advanced techniques suitable for experienced pool owners who are comfortable with chemical calculations and handling pool equipment. These methods allow for more accurate and proactive chemical adjustments, leading to a healthier and more enjoyable swimming experience.
For precise chemical balancing, accurate calculations are essential. This involves understanding your pool’s volume and the desired concentration of each chemical. Knowing your pool’s volume is the first step. You can usually find this information in your pool’s construction documents, or you can calculate it yourself using standard geometric formulas (length x width x average depth for rectangular pools, for example).
Once you know the volume, you can calculate the amount of chemical needed to adjust the levels.
Calculating Chemical Adjustments
Let’s say your pool holds 20,000 gallons of water and your test (using a reliable alternative method, such as a professional water test) reveals your free chlorine level is 1 ppm (part per million) while your desired level is 3 ppm. You need to add 2 ppm of chlorine to reach your goal. Assuming your chlorine source is 10% chlorine by weight (check your product label!), you can use the following formula:
Gallons of pool water x desired ppm increase x 8.34 pounds per gallon / percentage of chlorine in product = ounces of product needed
20,000 gallons x 2 ppm x 8.34 pounds/gallon / 0.10 = 3,336,000 ounces of product. Then convert to a more usable unit, like pounds: 3,336,000 ounces / 16 ounces/pound ≈ 208,500 pounds. This is a simplified example; you’ll need to consider the specific chlorine product’s concentration.
This calculation highlights the importance of using a precise measurement method. Always refer to the instructions on your specific pool chemical product for accurate dosage information. Never add chemicals directly to the pool without understanding the proper dilution techniques.
Using a Chemical Feeder
A chemical feeder offers a more controlled and consistent method of adding chemicals to your pool. These devices automatically dispense chemicals at a predetermined rate, maintaining consistent chemical levels. Different types of feeders exist, including liquid feeders, granular feeders, and tablet feeders, each suitable for different types of chemicals. Proper installation and calibration are crucial for effective use.
A step-by-step procedure for using a liquid chlorine feeder might look like this:
- Ensure the feeder is properly installed and connected to your pool’s circulation system.
- Carefully follow the manufacturer’s instructions for filling the feeder with the appropriate amount of diluted chlorine solution.
- Set the feeder’s dispensing rate based on your pool’s size, water flow rate, and desired chlorine level. This often involves experimentation and adjustment to achieve the optimal setting.
- Monitor your pool’s chlorine level regularly using an alternative testing method to fine-tune the feeder’s dispensing rate.
- Regularly clean and maintain the feeder according to the manufacturer’s recommendations.
Regular Water Changes and Chemical Balance
Regular partial water changes are vital for maintaining a healthy pool and reducing the burden on chemical balancing. Over time, contaminants accumulate in your pool water, regardless of how well you maintain chemical levels. These contaminants can interfere with the effectiveness of chemicals and lead to imbalances. Replacing a portion of your pool water with fresh water helps to dilute these contaminants and reset the chemical balance.
The frequency of water changes depends on several factors, including pool usage, climate, and the type of pool surface. A general guideline is to replace 10-20% of your pool water every 3-6 months. Larger water changes may be necessary in high-use pools or those with significant contamination issues. Remember to always balance the chemicals after a water change.
Troubleshooting Common Issues
Balancing pool chemicals without test strips relies heavily on observation and experience. While this method can be effective, it’s crucial to understand that problems can arise, and knowing how to identify and address them is vital for maintaining a healthy and enjoyable pool. Misinterpreting visual cues or neglecting preventative measures can lead to significant issues that may require more intensive intervention.Problems can stem from inaccurate visual assessments, environmental factors (like extreme weather), or simply the complexity of pool chemistry itself.
For example, cloudy water might be attributed to inadequate filtration, but could also indicate a chemical imbalance. Similarly, persistent algae might suggest insufficient chlorine, but could also point towards a pH issue affecting chlorine’s effectiveness. Understanding these potential overlaps is crucial for effective troubleshooting.
Cloudy Water
Cloudy water is a common visual indicator of a pool chemistry problem. Several factors can contribute to this issue. High levels of phosphates can fuel algae growth, leading to cloudiness. Insufficient filtration, caused by a clogged filter or inadequate pump runtime, can also result in cloudy water. Finally, an imbalance in pH can impact the effectiveness of flocculants, which are used to clump together fine particles for easier filtration.
Solutions include thoroughly cleaning the filter, increasing filtration time, ensuring adequate chlorine levels to combat algae, and adjusting the pH to the ideal range (7.2-7.8) to optimize flocculant performance. In cases of persistent cloudiness despite these measures, a professional pool service may be necessary.
Persistent Algae Growth
Algae thrive in environments with imbalanced chemistry. While a lack of chlorine is a primary suspect, other factors play a significant role. High pH levels reduce chlorine’s effectiveness, allowing algae to flourish. Similarly, low alkalinity can cause pH fluctuations, making chlorine less stable. Inadequate filtration prevents the removal of algae and debris, worsening the problem.
Solutions involve shocking the pool with a chlorine-based algaecide to quickly eliminate existing algae, adjusting the pH and alkalinity to the recommended ranges, and ensuring proper filtration. Regular brushing of pool walls and floors helps remove algae before it becomes established. Preventing sunlight from reaching the pool bottom can also help curb algae growth.
Scaling and Corrosion
Scaling (mineral deposits) and corrosion (metal deterioration) are often related to pH and alkalinity imbalances. High pH levels contribute to scaling, while low pH levels cause corrosion. High calcium hardness can exacerbate scaling, while low total alkalinity can lead to pH fluctuations that encourage both scaling and corrosion. Solutions involve carefully adjusting the pH and alkalinity to the ideal ranges.
Regular testing (even with alternative methods such as observing the rate of chlorine depletion) can help identify trends and prevent extreme imbalances. For existing scaling, specialized cleaning products might be necessary. For corrosion, consider replacing affected metal components as needed.
Preventative Measures to Minimize Chemical Imbalances
Regularly monitoring the pool’s visual appearance is the cornerstone of this approach. However, preventative measures are key to minimizing the need for reactive troubleshooting.
- Maintain consistent filtration: Run the pump and filter system for adequate hours each day, and regularly clean or replace the filter cartridges.
- Regularly brush the pool walls and floor: This helps prevent algae buildup and keeps the pool clean.
- Control debris: Regularly remove leaves, insects, and other debris from the pool to prevent them from contributing to chemical imbalances.
- Monitor weather conditions: Extreme heat or rain can significantly impact pool chemistry, requiring more frequent adjustments.
- Use a flocculant: A flocculant helps to clarify the water by clumping together small particles, making them easier to filter out.
- Consider a pool cover: A pool cover reduces water evaporation, minimizes debris entry, and limits sunlight exposure, reducing algae growth.
Illustrative Examples: How To Balance Pool Chemicals Without Using Test Strips
Let’s look at a real-world example of successfully balancing pool chemicals without relying on test strips. This method relies heavily on observation and a gradual approach, adjusting based on visual cues and the pool’s history.This example focuses on a homeowner who had a consistently clear, but slightly green-tinged, pool. They suspected an imbalance, but wanted to avoid the expense and potential inaccuracies of frequent test strip usage.
Instead, they opted for a careful, observational approach.
Successful Pool Chemical Balancing Without Test Strips
The homeowner began by observing the pool water daily, noting its clarity, color, and any unusual formations like algae growth or cloudy patches. The initial state showed a slightly murky, greenish hue, indicating a potential chlorine deficiency and/or an algae bloom. They started by adding a small amount of chlorine, about 1/4 of their usual dose based on past experience with the pool’s size and previous chlorine needs.
This wasn’t a precise measurement, but rather a cautious introduction. They waited 24 hours before making any further adjustments.Over the next few days, the homeowner observed subtle but significant changes. The green tint began to fade, replaced by a clearer, lighter shade of blue. The water became noticeably clearer. They continued to add small amounts of chlorine, observing the water’s reaction after each addition.
The process took approximately a week, with small chlorine additions every 24-48 hours, guided entirely by visual cues.
Visual Changes Observed During Balancing
Initially, the pool water displayed a slightly murky, greenish-blue color. There was a noticeable lack of sparkle, and the bottom of the pool was difficult to see clearly, even at a shallow depth. After the first addition of chlorine, there was no immediate drastic change, but over the next 24 hours, the green tinge began to lessen. The water started to become slightly brighter.As more chlorine was added in small increments, the green completely disappeared, giving way to a clearer, more vibrant blue.
The water became noticeably more transparent, with the bottom of the pool easily visible. The overall clarity improved significantly. The homeowner also noted a decrease in the amount of debris collecting at the bottom of the pool, indicating that the improved clarity wasn’t just an optical illusion but a reflection of a healthier water balance. There was no noticeable change in the water’s pH or alkalinity levels, as these remained stable throughout the process due to previous consistent maintenance.
Conclusion
Maintaining a sparkling, healthy pool doesn’t always require expensive test strips. By understanding the visual cues of chemical imbalances and utilizing alternative testing methods, you can achieve a perfectly balanced pool with confidence. Remember, consistent observation, proactive scheduling, and a bit of know-how are key to success. This approach not only saves you money but also empowers you to become a true pool chemistry expert, ensuring your swimming oasis remains pristine and inviting all season long.
FAQ Summary
What if my visual cues are unclear or contradictory?
If you’re unsure about the visual indicators, it’s best to err on the side of caution and use a liquid reagent test kit for a more precise reading. Don’t hesitate to consult a pool professional if you’re still uncertain.
How often should I check my pool’s chemistry using alternative methods?
The frequency depends on factors like weather, pool usage, and water evaporation. Aim for at least once a week, more frequently during hot weather or heavy use.
Can I over-correct chemical imbalances using this method?
Yes, it’s possible. Start with smaller adjustments and observe the results before making further additions. Always follow the instructions on your chosen chemical products carefully.
What should I do if I suspect a major chemical imbalance?
For significant issues like persistent algae or severe scaling, it’s best to consult a pool professional for assistance. They have the expertise and equipment to address more complex problems.










